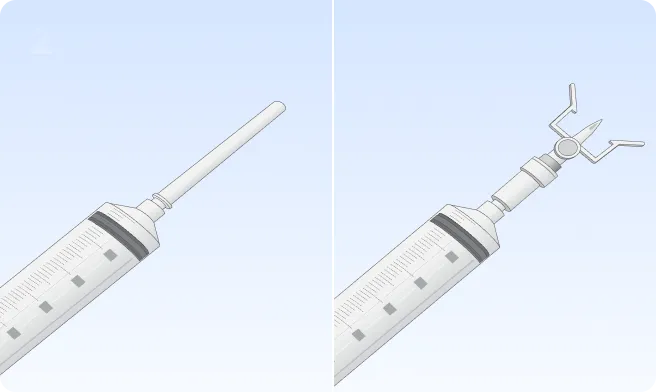
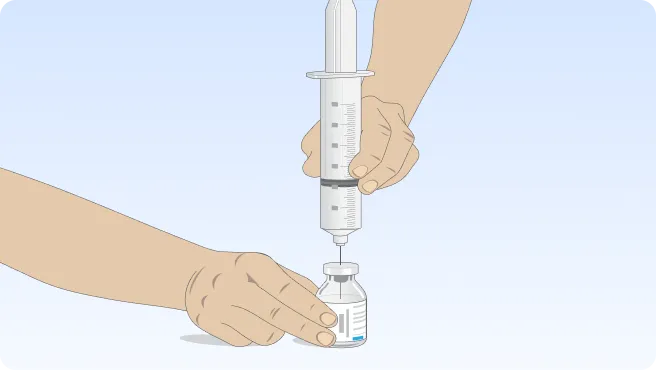
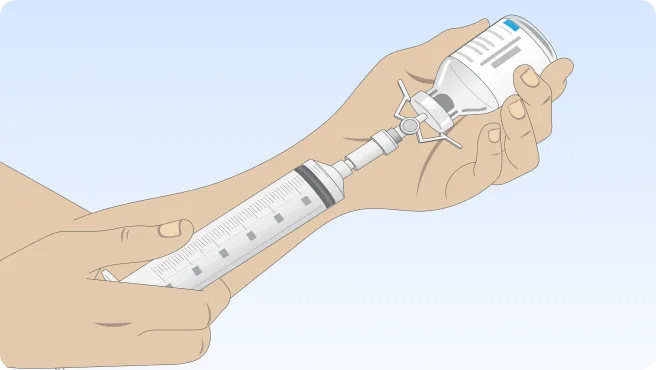
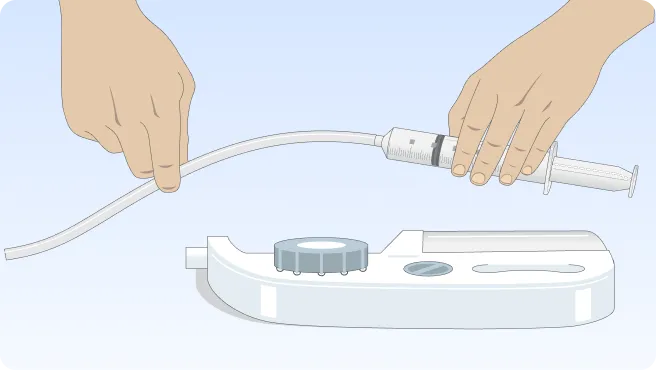
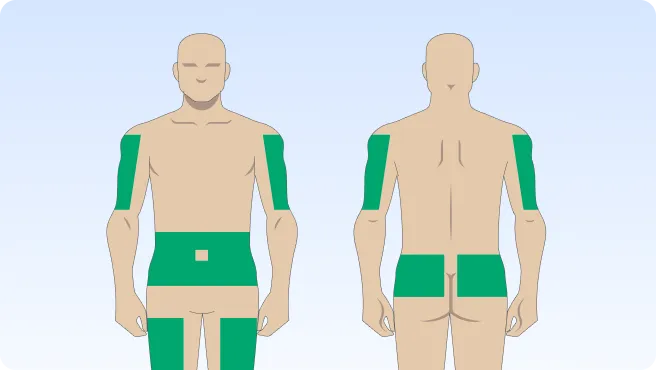
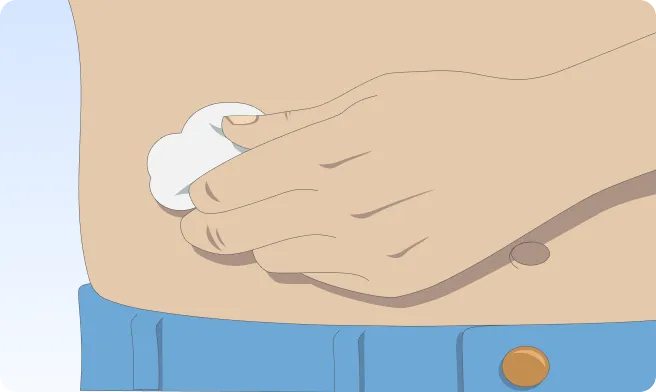
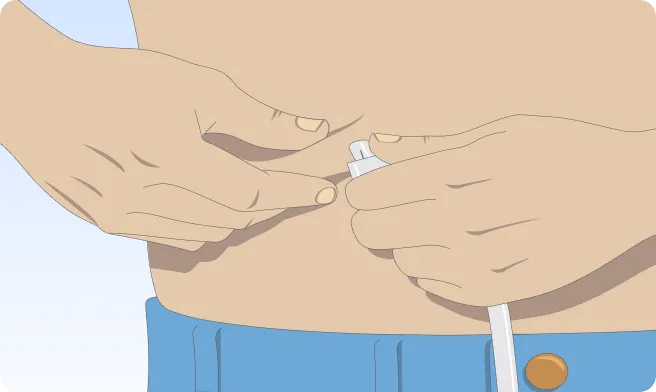
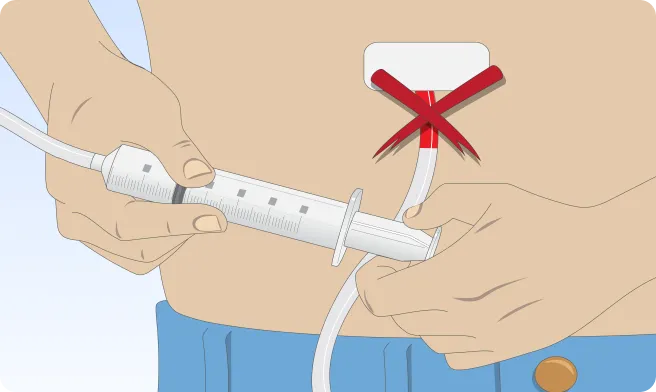
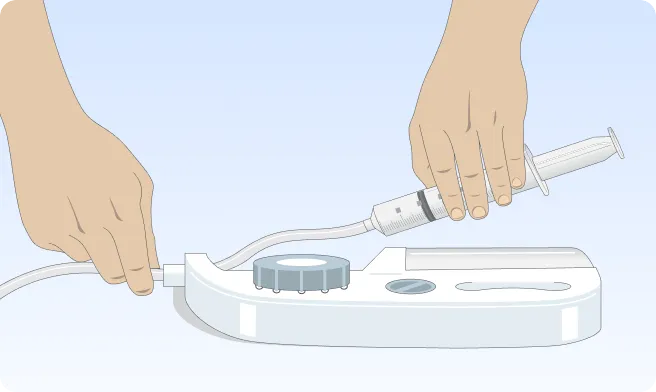
Follow the step-by-step instructions on how to infuse belowBefore the infusion
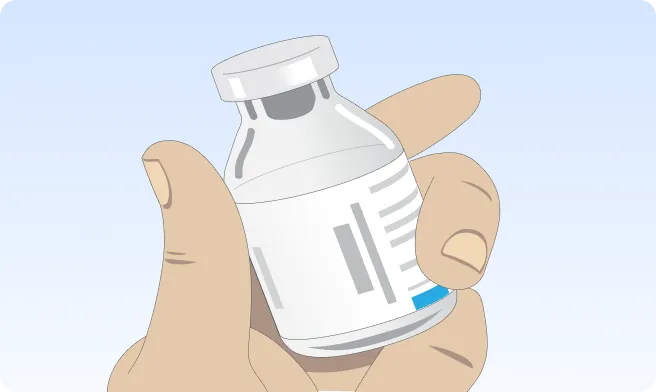
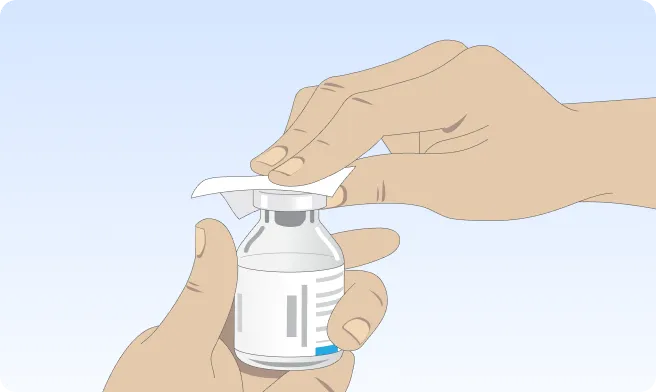
If the vial(s) of XEMBIFY is refrigerated, remove from the refrigerator prior to your infusion and allow the vial(s) to reach room temperature. This may take 60 minutes or longer. DO NOT apply heat to the vial(s) or place in the microwave. Instead, let the vial(s) warm to room temperature naturally.2